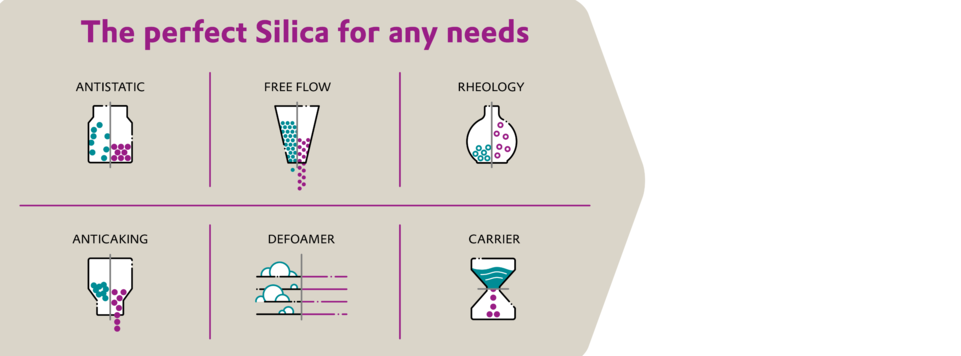
THE SILICA SPECIALISTS AT EVONIK
At Silica what drives us is the ablity to improve nearly every product in every market we serve. That’s why we give three promises to really inspire our customers:
Expertise, Trust and Value.
The Silica specialists at Evonik. Inside, to get it right. ... MORE